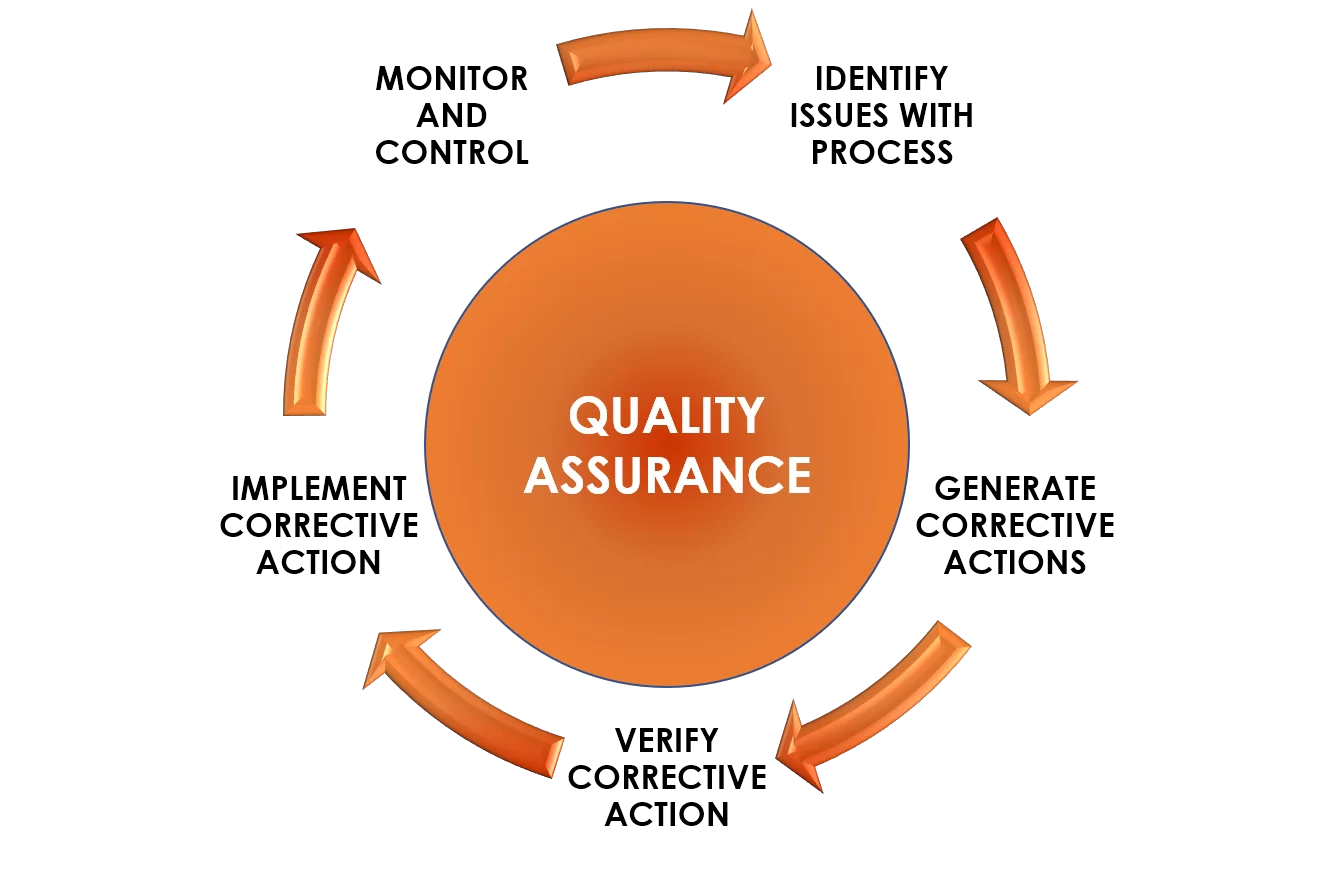
At RADICON'S ‘quality’ is the basic foundation on which products are developed and produced. At every stage of the production cycle, quality stems from strict adherence to GLP/cGMP requirements. Providing support are the latest international quality systems incorporated from concept to commercial production. The quality assurance team has developed systems to ensure that quality is built in to the process, leading to consistency of quality each time, every time.
Assurance of product quality originates from strict adherence to cGMP systems, qualification of material vendors, stringent in-process controls, and effective change control and document control systems. All equipment, facilities, products, processes and cleaning procedures are completely validated and documented. The RADICON'S quality assurance team employs the world’s most sophisticated analytical and quality control instruments. Besides, all personnel undergo training and development on cGMP, safety protocols, health & hygiene and other various job-associated activities. Periodic self-audits ensure compliance to cGMP. Approvals obtained from the Ministry of Health, along with the ISO certification stand out as stellar examples of RADICON'S testimony to quality. The ISO 9001:2000 certification received by RADICON reflects the quality management system that encompasses formulation development, manufacturing and marketing of pharmaceutical dosage forms such as tablets, capsules, ointments , injections, jelly based ointments etc.
